Definition of Drop Electrochemistry
Drop electrochemistry is the development of electrochemical measures at a drop level. The development of this kind of technique allows for the study of a particular phenomenon of great interest.
Application of Drop Electrochemistry
Electrochemistry is in our daily life: from batteries in our devices to the chemical reactions in our bodies, in redox reactions with chemical energy converting to electrical energy and vice versa. Also, electrochemistry has wide applications in industrial technologies like sensors, biosensors, electroplating processes, and wastewater treatment among others.
Typically, the cells used in electrochemistry consist of a volume of milliliters with three electrodes, the working electrode (graphite, gold, etc.) where the interest reaction/detection occurs, reference electrode, and counter electrode (Rackus, Shamsi, & Wheeler, 2015).
With the development of small electrodes such as printed electrodes and microelectrodes, it is possible to work with electrochemistry at a microliter level.
Currently, several applications using electrochemistry at a microliter level have been developed:
- Production of organic compounds
- Analytical methods
- Catalytic activity assessment
- Scanning of surfaces
The implementation of these applications requires a high precision of microfluidic control: such precision can be achieved by using syringe pumps which allows low flow rates in the picoliter range.
Research and Discoveries
Drop electrochemistry has been used in biological systems with different purposes. A few key research findings are below:
- The Limoge group has reported the use of small drops for enzyme kinetic studies with electrochemical probes like FcMeOH and [OsII(by)2 pyCl]+ which are electrochemical mediators for Horse Radish Peroxidase catalysts (Challier et al., 2013). These electrochemical studies were performed in the drop range of 15-50 µl. With the Chemyx Fusion 100, it is possible to deliver small drops in the range of 0.5 µl. Fundamental studies like this allowed for the development of new biosensors.
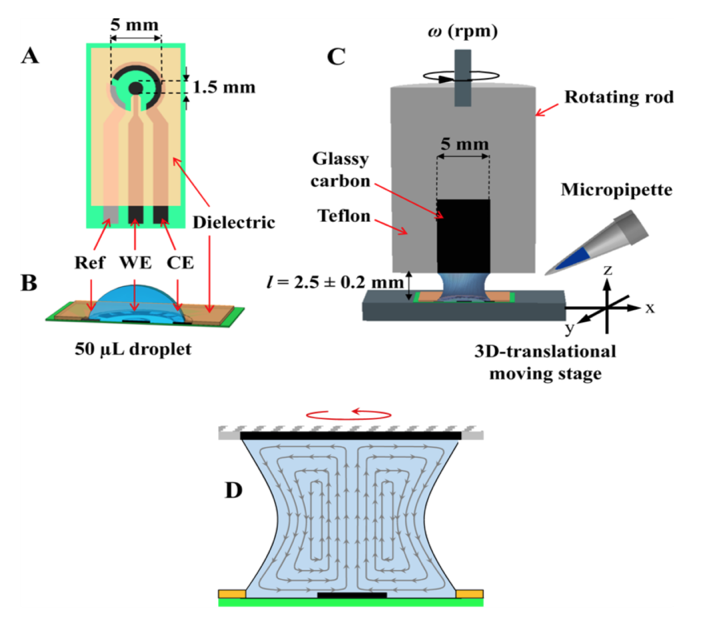
Fig1. A) Three electrode setup in a drop, B) Rotatory Disk adapted to the drop, C) Fluid dynamics generated by rotation (Challier et al., 2013).
- As a result of analytical methods, Kurita et al. (2007) developed a microfluidic system for the evaluation of the affinity and catalytic activity of biomolecules in an electrochemical surface plasmon resonance flow cell at a flow rate of 20µl/min. This system allows the evaluation of the performance of bifunctional biomolecules such as ribozyme or abzyme that can have therapeutic applications like in anticancer drugs (Kurita, Yokota, Ueda, & Niwa, 2007). It has also been implemented in the study of individual reactions and for the production of microarrays; the arrangement of small drops is also referred to as discrete microfluidics and has relevance in the study of kinetics and biosensor development (Lindsay et al., 2007).
- In other systems, drop electrochemistry or meniscus electrochemistry will help to deliver compounds on the top of pipette ultramicroelectrodes and follow the progress of electrochemical reactions. In this configuration, small defects in new advanced materials, such as graphene layers, can be analyzed. The low rate of liberation provided by a syringe pump can be used to stimulate small groups of cells and study with ultramicroelectrodes. The meniscus electrochemistry of drop-electrochemistry is perfect for this purpose.
- Unwin group has developed a study to determine the kinetics of TPMCl (triphenylmethyl chloride) hydrolysis by the implementation of an interface 1,2-dichloroethane/water. A syringe pump was used to control the expansion of the drop at a selected rate to merge the two phases and calculate the Cl- production due to the hydrolysis process (Barker et al., 1999).

Figure 2. Drop electrochemistry setup for kinetic studies using a syringe pump (Barker, Gonsalves, MacPherson, Slevin, & Unwin, 1999)
- With compound production, electrochemical methods are advantageous since the formation of side products diminishes due to not requiring reducing or oxidizing agents. The use of microreactor systems has been developed and successfully employed in the production of different compounds. In these setups, syringe pumps were employed with flow rates as low as 40µl/min (Watts, Gattrell, & Wirth, 2011).
The study of surfaces is also an important topic because of their application in industrial processes. The conjugation of Scanning Electrochemical Microscopy (SECM) in a drop level has proven to be a powerful tool for the study of catalysts, surface corrosion, and biological processes. Cortes-Salazar et al (2010), developed a fountain pen probe incorporating a fluidic microchannel with a syringe pump to extend the scope of SECM to the study of dry surfaces in a contact regime to overcome the solvent evaporation problem (Cortes-Salazar et al., 2010).
Conclusion
- In general, drop electrochemistry allows for the characterization of complex reactions by kinetic studies that need a high fluid dynamic control with chemical and biological relevance. The rate of microfluidic or drop size control can be achieved by the implementation of the correct syringe pump. Several setups can be designed as Nanoscale Pipetting coupling to a syringe pump to study small arrays of water or even new catalysts (Rodolfa et al., 2006).
References
- Barker, A. L., Gonsalves, M., MacPherson, J. V., Slevin, C. J., & Unwin, P. R. (1999). Scanning electrochemical microscopy: Beyond the solid/liquid interface. Analytica Chimica Acta, 385(1–3), 223–240. https://doi.org/10.1016/S0003-2670(98)00588-1
- Challier, L., Miranda-Castro, R., Marchal, D., Noël, V., Mavré, F., & Limoges, B. (2013). Kinetic rotating droplet electrochemistry: A simple and versatile method for reaction progress kinetic analysis in microliter volumes. Journal of the American Chemical Society, 135(38), 14215–14228. https://doi.org/10.1021/ja405415q
- Cortes-Salazar, F., Lesch, A., Momotenko, D., Busnel, J.-M., Wittstock, G., & Girault, H. H. (2010). Fountain pen for scanning electrochemical microscopy. Analytical Methods, 2(7), 817–823. https://doi.org/10.1039/C0AY00096E
- Kurita, R., Yokota, Y., Ueda, A., & Niwa, O. (2007). Measurement in a Microliter Volume Flow Cell for Biomolecules, 79(24), 9572–9576.
- Lindsay, S., Vázquez, T., Egatz-Gómez, A., Loyprasert, S., Garcia, A. a, & Wang, J. (2007). Discrete microfluidics with electrochemical detection. The Analyst, 132(5), 412–416. https://doi.org/10.1039/b617631c
- Rackus, D. G., Shamsi, M. H., & Wheeler, A. R. (2015). Electrochemistry, biosensors and microfluidics: a convergence of fields. Chem. Soc. Rev., 44(15), 5320–5340. https://doi.org/10.1039/C4CS00369A
- Rodolfa, K. T., Bruckbauer, A., Zhou, D., Schevchuk, A. I., Korchev, Y. E., & Klenerman, D. (2006). Nanoscale pipetting for controlled chemistry in small arrayed water droplets using a double-barrel pipet. Nano Letters, 6(2), 252–257. https://doi.org/10.1021/nl052215i
- Watts, K., Gattrell, W., & Wirth, T. (2011). A practical microreactor for electrochemistry in flow. Beilstein Journal of Organic Chemistry, 7, 1108–1114. https://doi.org/10.3762/bjoc.7.127

